Flash Point Temperature – What is it?
The Flash Point of a substance or material is the “lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapors in a quantity such as to be capable of forming an ignitable vapor/air mixture,” according to the British Standards classification, EN 60079 Part 10-1, which covers the classification of areas where flammable gas or vapor hazards may arise.
Let’s clear up some common misconceptions. The Flash Point Temperature is not the same as the temperature at which autoignition occurs. Autoignition is the temperature that causes spontaneous ignition without an ignition source, a principle you may be familiar with in the context of diesel or compression ignition engines.
It should not be confused with the Fire Point Temperature, the temperature at which the vapors continue to burn for at least five seconds after ignition or after the ignition source is removed.
As a rule, the Fire Point Temperature is higher than the Flash Point Temperature, and the Autoignition Temperature significantly higher than even the Fire Point Temperature.
For combustion to be sustained following ignition, sufficient vapors need to be generated, and at the Flash Point Temperature, this is not the case, but this is so once the Fire Point Temperature is reached.
Flash Point Temperature and Hydro-Carbons – Why is it Important?
One of the primary uses of flash point testing is assessing the safety hazards, and consequently the flammability, of liquids and semi-solids, or in this case, fuels, oils, and greases. This then allows the classification of these materials according to the result.
The lower the Flash Point Temperature, the higher its risk of flammability.
For instance, fuels with a Flash Point Temperature of less than 37.8°C or 100°F are classified as flammable, while those with a higher Flash Point Temperature are classified as combustible.
That is why, with oils and greases, the Flash Point Temperature is quoted under “Section 9: Physical and chemical properties” of the lubricant’s accompanying Safety Data Sheet (SDS) and, in the case of oils, also typically shown in the Product or Technical Data Sheet (PDS/TDS).
Flash Point Temperature Testing Methods – What Can Go Wrong?
According to Anton Paar, a seller of laboratory instrumentation, the following definition of Flash Point Temperature that is widely used in standard test methods is: “The lowest temperature of the test specimen, corrected to a barometric pressure of 101.3 kPa, at which the application of an ignition source causes the vapor of the test specimen to ignite momentarily and the flame to propagate across the surface of the liquid under the specified conditions of the test.”
Please note that the value of the flash point is not a physical constant but the temperature measured for a fuel or oil. This means it’s highly dependent on the instrumentation and procedure used.
However, with the right equipment and adherence to a standardized test method, you can ensure comparable results and the accuracy of the reported values.
Factors that can affect the test result include:
- the choice of equipment,
- the test method and any variation to the protocol,
- the temperature ramp rate on automated testers,
- time allowed for the sample to equilibrate,
- sample volume,
- and whether the sample is stirred.
Therefore, as with all other tests, adherence to a standardized test method is required to ensure comparable results and the accuracy of the reported values.
Several test methods can be followed depending on the substance being tested, such as fuels, solvents, or lubricating oils, and for varying reasons, such as spilled versus contained stored product.
The choice of test method is one such issue that arises as some lubricant PDS/TDS may show differing test methods, namely, Open Cup and Closed Cup.
In addition, Flash Point testers may be no-equilibrium or equilibrium.
Non-equilibrium refers to the fact that the ignition source is applied at intervals as the test liquid is heated. Still, the vapor temperature may differ from the liquid or not in temperature equilibrium.
Equilibrium refers to the point where the sample liquid and its vapor are in temperature equilibrium, as both have the same temperature.
Open Cup Flash Point Testing – ASTM D92-18
ASTM D92-18—Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester is the proscribed method for testing the Flash and Fire Point Temperatures of petroleum products with Flash Points above 79°C (175°F) and below 400°C (752°F) except fuel oils.
In the open cup test, an ignition source is passed horizontally over the surface of the liquid while the liquid and cup are being heated. Typically, the temperature is raised in incremental steps until a brief flash occurs, and the temperature at which this happens is recorded as the Flash Point Temperature. The required volume for testing is around 100mL, and the test time is 30 minutes, depending on the value reached.
The most common method of open cup testing is the Cleveland Open Cup (COC), upon which ASTM D92-18 is based, although other apparatuses such as Tag and Setaflash exist.
However, variations in the height of the ignition source above the liquid will result in varying measured values, and it is possible that with sufficient height above the liquid, the flash point and fire point temperatures will coincide.
In addition, the problem with open cup testers is that the vapors produced by heating the test liquid are free to escape to the atmosphere, and conditions within the laboratory can cause variations. As a result, temperatures above ambient may result in higher flash point readings because of the reduced concentration of vapors in the warmer air.
The original reason for this test was to assess the potential hazards when there were spillages of liquids.

Figure 1: The Anton Paar CLA 5 Cleveland Open Cup Flash and Fire Point Tester.
Closed Cup Flash Point Testing – ASTM D93-20
ASTM D93-20 – Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester is the proscribed method for testing the Flash Point Temperature of petroleum products with Flash Point Temperatures ranging from 40°C to 370°C.
ASTM D93 refers to testing via the Pensky-Martens, but the standard is split into three procedures: A, B, or C.
Procedure A is for distillate fuels and new and in-service lubricants. Procedure B applies to used lubricating oils or to oils of such kinematic viscosity that they are not uniformly heated under the stirring and heating conditions in Procedure A.
In the closed cup test, the sample, usually 75mL, is tested in a sealed chamber and, therefore, not affected by atmospheric conditions, and the flash is detected electronically. This mimics the situation of an ignition source in a sealed container such as a tank or other storage vessel, which is why the Closed Cup Method is often reported in the SDS for a lubricant.
The results obtained from the Closed Cup test are typically lower than those of the same product tested in the Open Cup method, as the test vessel contains heat that is more likely to make the test sample flammable at an earlier stage.
Other Closed Cup test apparatuses besides the Pensky Martens include Abel, Tag, and Setaflash.

Figure 2: The Koehler Automatic Pensky-Martens Closed Cup Flash Point Tester in the closed and open positions to show the actual cup.
Flash Point Temperatures and New Lubricant Management – What Can It Tell Me?
The two main test methods will be discussed to stay focused on lubricants: Cleveland Open Cup and Pensky-Martens Closed Cup, respectively, as per ASTM D92 and D93. In addition, there are also other test standards such as those from the International Petroleum (IP) and the International Standards Organisation (ISO):
- IP36 and ISO 2592:2017 for Cleveland Open Cup
- IP 34, ISO 2719:2016 for Pensky-Martens Closed Cup
For health and safety personnel, the Flash and Fire Point values are essential in determining the correct storage of lubricants in work areas, stores, and vessels such as tanks.
As stated, the value helps to determine the flammability of the lubricant whereby:
- The closed cup result best mimics the conditions of lubricants stored in drums and tanks.
- The open cup result indicates the flammability of spilled lubricants into the bunds.
For the engineer, the Flash Point values indicate the flammability of lubricants within working machinery in applications such as engines, turbines, and compressors where higher running temperatures are experienced.
In this instance, either result may be important since engine sumps are often under a vacuum due to the crankcase breathing via the inlet manifold, which would reduce the concentration of vapors. At the same time, sealed tanks on turbine trains may be subject to vapor concentrations unless purged.
For this reason, anti-static additives are often used in turbine oils as static discharge is an ignition source.
This happened on one of the oil rigs in the Brae Field in the North Sea, where a static discharge was deemed to cause an explosion within the turbine tank, which fortunately held but caused the top of the tank to deform.
This information is reported in the SDS and the PDS/TDS, with the Health and Safety personnel focusing on the SDS and other crucial safe handling information. At the same time, the engineer will no doubt view the PDS/TDS and other important physical, chemical, and performance characteristics when selecting an oil.
Flash Point Temperatures and Used Oil Analysis – What Other Information Can It Provide?
As the flash point depends on the vapors produced, any contamination of lubricating oil by a fluid of lower viscosity and more flammable nature will drop the reported Flash Point Temperature. In conjunction with a Kinematic Viscosity test, the Flash Point test can help judge whether a drop in viscosity is due to some other factor, such as cracking by overheating or contamination by a solvent or fuel.
Therefore, one of the most common applications where Flash Point testing is done is on diesel engine lubricants where a drop of more than 20°C from the new oil value on the Closed Cup value is considered a problem owing to the likely presence of Light Fuel Oil (LFO) or petrol/gasoline.
Flash Point Testing by Small Scale Closed Cup
A pass/fail result is generally given for the test in most applications. In the Small Scale Closed Cup procedure (ASTM D3828 – Method A), only 2mL to 4mL of sample is required, which is beneficial where small sample bottles are in use.
More importantly, in a laboratory, the Small Scale Closed Cup test takes a lot less time, around 2 minutes, to obtain a result than the typical 30 minutes or more to measure by ASTM D92 or D93. With today’s bio-blended fuels, ethanol can also be detected.
However, one of the biggest problems in using this method for fuel dilution testing is the safety aspect in the laboratory, where heating of fuel-laden samples may be a risk. For this reason, end-users submitting samples suspected to have some dilution should forewarn the laboratory.
Careful adherence to the procedure is required for consistency of results. Other possible negatives include excessive coolant leakage in the oil, which may give a false pass as the coolant would impact the vapor formation. In addition, bio-diesel, as with heavy fuel oil (HFO), is generally heavier, so it cannot be detected.
Most laboratories choose to provide a pass-or-fail result because while the actual drop in the Flash Point can be correlated to the percentage of fuel dilution, this will vary depending on the fuel and oil types. In addition, the amount of time a sample is exposed to the atmosphere can impact the resulting value, as lighter fractions could be lost before testing.

Figure 3: The Stanhope-Seta Setaflash Series 3 instrument for Small Scale Closed Cup testing.
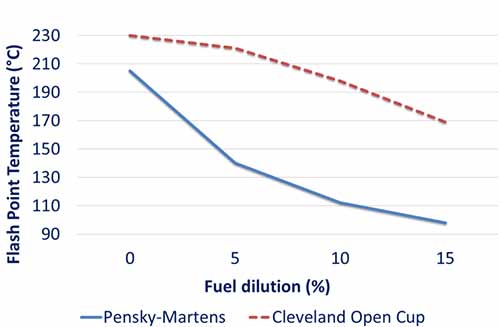
Figure 4: A correlation between Flash Point Temperature and Fuel Dilution as a percentage.
Figure 4 illustrates that the closed cup method produces lower results than the open cup method. Again, regarding detecting fuel in the oil, the closed cup method shows a more obvious drop at levels below 5% than the open cup for the correlation.
Case Study of Using Flash Point Temperature Testing for Fuel Dilution
One instance where flash point testing for diesel fuel dilution was put to good use was in the North Sea, where access to land-based laboratories is restricted.
The standby engines on the oil rigs are meant to be run for at least one hour once a fortnight to ensure that all contamination from condensation and fuel dilution is burnt off. However, the operators often only ran the engine for ten to fifteen minutes.
This resulted in much higher levels of fuel dilution than would have been the case had they been run properly. As most oil rigs have a laboratory with a flash point tester, the lubrication technician would test the oil and then confront the operators where excessive fuel dilution was found.
This resulted in a drop in reported fuel dilution from the routine sampling, with the consequent safety benefits should the engines need to be used in anger. Unfortunately, the situation has changed since environmental personnel insisted that the engines only be run for ten minutes to minimize emissions.
What Else Can a Used Oil Flash Point Test Highlight?
One correlation to the flash point value is viscosity. Generally, the higher the viscosity, the higher the flash point value. It can often be seen on a PDS for a gear oil range that as the viscosity increases, so does the Flash Point temperature.
Consequently, where fuel or solvent material may not be a risk, a drop in flash point could indicate base oil cracking resulting from various causes.
Another possible source of lighter fractions might be topping up with the wrong oil of a lower viscosity grade, which introduces lighter fractions.
Flash Point Temperature values will always be quoted for new lubricants given the potential safety aspects of stored or spilled/leaked product. In addition, given the conditions of some equipment operating at higher temperatures, the Flash Point value will remain a key element in the engineer’s selection of a lubricant.
As to the value in used oil analysis, the potential is primarily in the possible fuel dilution of engine oils or solvent contamination. However, there are a variety of primary fuel dilution tests with other secondary confirming tests, and generally, with solvents, the likelihood will be a lowered viscosity resulting from that contamination.
Consequently, given the time and volume required for ASTM D92 and D93, most laboratories will not necessarily undertake this test and will more likely undertake the Small Scale Cup test and offer a pass/fail result.
In addition, the consistency of the results will also depend on the adherence to the test procedure and the care and consideration given to the sample handling and collection.









