Why The Most Popular Oil Analysis Test Ever Might Actually Be the Worst
Over the years, oil analysis has gone from something adopted by just a few asset owners with critical components to a universal tool to achieve reliability.
As oil analysis entered the mainstream, laboratories emerged to serve this growing demand, in many cases using or developing innovative and robust analytical techniques to unlock the information hidden in each sample submitted.
As efforts evolved to standardize and create uniform approaches to analysis, the end-user community began to expect the advantages of competitive pricing to get maximum value for their investment in oil analysis services.
Of course, there is nothing wrong with pursuing value for your money. Still, perhaps somewhere along the line, the commercial lab industry began to bend a bit to the demands of the purchasing agents pushing for lower-cost analysis solutions.
After all, you can have the best lab in the world, but no one will benefit if you don’t win any purchase orders. But whether an asset owner demands a highly detailed and outcome-oriented test slate or seems to be looking to perform something called “oil analysis” for the lowest possible per-sample price, there may be one test that seems to end up on everyone’s test slate and is perhaps the most confusing, subjective, inaccurate, and useless test ever devised for oil analysis – the Crackle Test.
Do I Have a Water Problem?
The lowest common denominator for oil analysis may be wear condition determination. Everyone seems interested in the asset’s condition, the more significant value component of the machine-oil state.
But it is almost universally recognized that one of the most common and troubling contaminants that can derail an otherwise healthy machine, and its lubricant, is the presence of water. “If only there were a simple (and cheap) way to know whether I need to be worried about water” seems to be the sentiment and statement that would lead to a resourceful person suggesting that yes, we can do this with a simple laboratory hot plate.
Albert Einstein said, “Everything should be made as simple as possible, but not simpler.”
In truth, his quote was more complex, ironically, and referred to irreducible basic elements and without having to surrender the adequate representation. The latter draws concern when you carefully consider the method and value of the crackle test carefully.
A solution to the “Do I have a water problem” question must address what a water problem is, how water impacts the machine in question, how water impacts the lubricant it is occupying, what are the minimal values at which an impact is felt or measurable, and the alerting and alarming levels at which we wish to monitor the water concentration.
An asset owner would likely want to believe that at water concentrations below the lower limit of detection of the method, they need not worry about water’s impact on their asset or its lubricant.
Or, a second and more defining analysis test can be triggered using the screening technique with high confidence and little chance that a potentially damaging condition would go undetected.
How Low Can the Crackle Test Go?
Because the Crackle Test does not have an associated ASTM Standard, we cannot rely on the precision statement that such a method would supply. Therefore we must look at what the published literature says about the technique or how the performing laboratories represent their particular version of the method.
One published article states, “Under carefully controlled lab conditions, the crackle test is sensitive to around 500 ppm (0.05 percent) of water-in-oil depending on the type of oil.”1
Another source states, “But as you migrate away from engine oils into industrial oils, the 500 ppm detection limit is no longer valid in all oil types; specific oil types exhibit different crackle detection limits.”2
This last article outlines a study by the authoring lab that determined that with varying lubricant types and used lubricant conditions when compared to accurately determined values (Karl Fischer method), the detection varied from a lowest positive limit of 100ppm to a highest negative at 1830ppm. This means that sometimes oils can be contaminated with as much as 0.15% moisture or higher and be undetected by the crackle test.
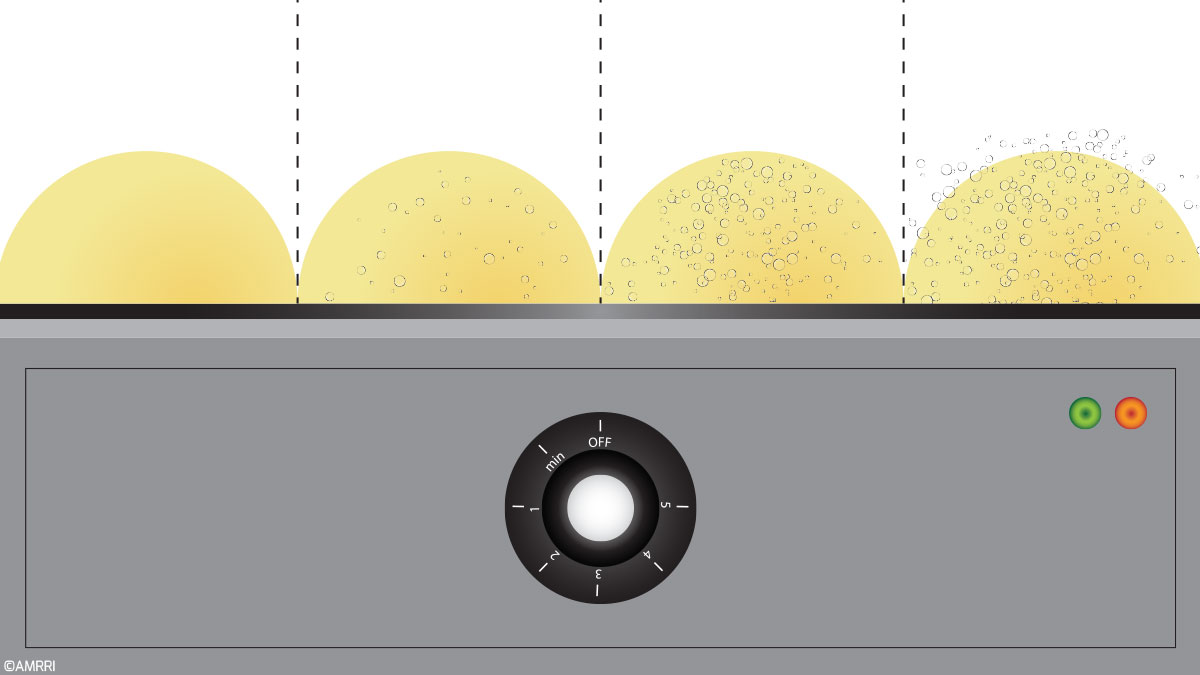
To look at this myself, I selected several samples from our lab that had already been tested to ppm levels and subjected them to a procedure for Crackle Test.
This procedure used a small hot plate with a white ceramic surface with only minor staining from use, using the controller setting at 170° C. At this setting, I measured the surface temperature to be approximately 320° F. (Let’s talk about temperature accuracy and stability in a moment).
I checked five samples with measured moisture levels ranging from 48 ppm to 2412 ppm, as outlined in the table below. Two of these samples were below the 500 ppm threshold, usually cited as the lower limit of detection, while the other three were well above this value.
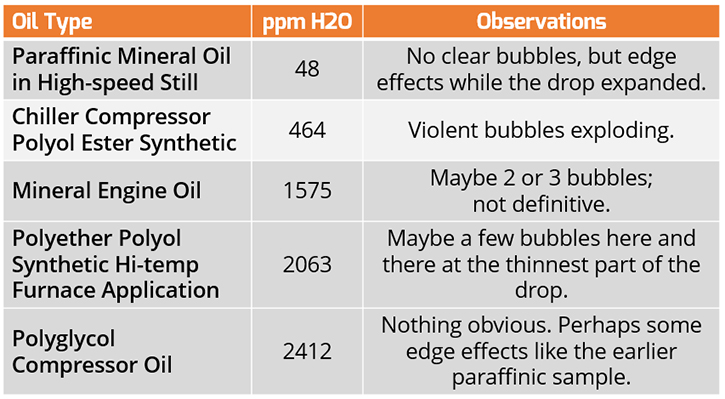
Figure 1. Crackle Test Results
Of these, the only sample that would have an obvious and clearly observed “Positive” Crackle Test result was the Chiller compressor sample, with only 464 ppm H2O as the measured result.
This positive result is undoubtedly from the refrigerant absorbed into the lubricating oil. There was no clear threshold to screen these samples for further analysis in this population of varying types of synthetics and mineral oils.
How Low SHOULD Your Moisture Level Test Be Able to Go?
So even if the Crackle Test could reliably detect and screen samples above and below 500 ppm, with the method here or some enhanced approach, is it good enough for screening purposes?
One of the key reasons to detect and quantify moisture contamination is the potential impact on bearing life. Figure 2 below shows a relationship between moisture level and life impact for rolling element bearings based on the disruption of an effective EHL film in the presence of higher moisture levels.
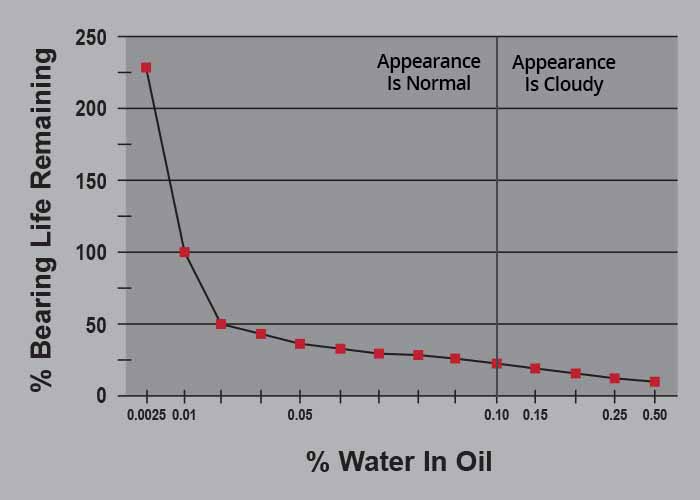
Figure 2
This chart shows a cut-off line of 1000 ppm H2O, or 0.1%, as the “observable limit.” Even if we assume that we can reliably detect moisture down to 500 ppm, this chart shows us that there is a 60% reduction in life for these bearings when moisture levels are maintained at that level.
With a focus on machinery life and sustainability, should we be content with employing monitoring techniques that, under the best of conditions, only begin to alert us to the presence of a contaminant that can be robbing us of 60% of the life and thus the investment in this capital asset?
If asset owners truly understood this limitation of the method, I doubt that it would be approved as a screening test for monitoring their machinery.
My experience as an asset owner responsible for lubricant monitoring included standby nuclear power plant components that were expected to be kept in a state of readiness, even when they were not routinely operated to support the plant.
In one such location, we employed housekeeping and cleanliness standards, along with motor heaters that would keep the oil warm in standby condition to prevent the buildup of moisture in the oil.
In this state, the monthly samples routinely returned very low moisture levels as measured by the Karl Fischer Test of 20-40 ppm moisture. So when an oil sample I took rose to 85 ppm, I took notice.
A review of the motor manufacturer and lubricant supplier guidelines showed an operating limit of 1000 ppm moisture. So while there was no “condemning limit” in play, the clear increase in moisture raised concerns that were only detectable due to the routine and precise trending of the ppm moisture levels.
Because a primary concern was the integrity of the bearing housing water cooler as the source of this moisture, a hydro-test pressure check was performed on the cooler. A leak was found that was calculated to increase at a significant rate when the motor would be called for an extended run, and by finding the low level of water contamination, a significant issue was avoided.
So Really, What IS the Crackle Test Method?
It seems like an easy question to answer, but with testing, if no standard exists, no clear guidance is there for the correct and consistent way to measure. For example, a search of published literature produces the following hot plate temperatures for performing the test:
300° F, 320° F, 400° F, and 600° F
I was shocked to find 600° F published as a temperature to use for the Crackle Test, and I now believe that someone mistakenly converted 320° Celcius to Fahrenheit.
Still, it underscores the problems with a method with no agreed-upon standard. Even the variation from 300 to 320 to 400° F could significantly vary results.
What about the hot plate? Most methods say to use “approximately 320° F,” for example, perhaps recognizing that hot plate temperatures are not precisely controlled. In fact, a hot plate is typically an on-off thermostatically controlled heat source, generally requiring moderation with a target volume of liquid being heated.
Suppose one watches a hot plate surface over time. In that case, the temperature will dip low below the target temperature, then turn back on and cause the temperature to rise above the target fairly significantly.
For my tests in this article, while attempting to maintain a hot plate center temperature of 320° F, a variation was seen from 308.4 to 327.8° F. The hot plate temperature is not uniform across the hot plate.
An infrared image can show that the hot plate is hottest in a pattern where the surface is closest to the underlying heating element. Another factor is the amount of oil added to the hot plate and how quickly.
When a drop of oil touches the hot plate, the oil itself has a cooling effect on the surface, and it will take some time for the surface temperature to heat back up. So there are many limitations to truly ensuring that the heat from a hot plate is sufficient and uniform and that the moisture in the sample is heated to or close to that target temperature.
And the Value of the Crackle Test Is …
To say that the Crackle Test provides no value is not accurate.
For something to provide no value, it should leave you with the same information you had before you performed the test. I will argue that the Crackle Test is worse than a test of no value.
The Crackle Test is harmful. It gives the asset owner a false sense of security that moisture is not a problem when a “negative” result is received. The only validity for a screening test is that it must reliably tell us when additional testing is required.
The Crackle Test fails to meet this requirement by allowing samples contaminated with water to escape further scrutiny: the follow-up and accurate testing options such as Karl Fischer or VaporPro (ASTM D6304 and D7546) could find and accurately quantify these potential problems.
The argument is typically that the Crackle Test is just a screening test, a go-no-go gauge to see if further testing is warranted. A field test that can find the worst-of-the-worst to prioritize action.
But if the test itself cannot be relied upon to inform us when additional testing is required consistently, it has no value. It should not be found in a laboratory test slate when protection against the damage of water contamination is needed for any critical lubricated asset.
References
- Noria Corporation, “Monitor Water-In-Oil with the Visual Crackle Test”, Machinery Lubrication, March 2002.
- Eurofins, “Crackle Test: Do You Know Your Detection Limits?”, Data Interpretation, Program Management, Routine Testing, https://testoil.com/program-management/crackle-test-do-you-know-your-detection-limits/ May 2011.