Zinc (also known as ZDDP, ZDTP or zinc dialkyldithiophosphate) is the most common lube oil additive. Patented in 1944, this molecular family has been used as low-cost, multifunctional additives in engine oils, transmission fluids, hydraulic fluids, gear oils, greases, and other lubricant applications. ZDDP’s’ unique ability to act as an antiwear agent, an effective peroxide decomposer that protects against oxidation and corrosion and a mild extreme-pressure (EP) agent is what makes it the lubricant formulator’s best friend.
But lately, its popularity has been under attack. The rise of emissions regulations, exhaust after-treatment systems and new industrial lubrication requirements are driving formulators to alternative strategies. But finding replacements for a technology that has served the industry well for 80 years is complex, and end-users of lubricants will need to adapt their understanding of common lubricant degradation behaviours as formulations change.
The Basics of Zinc in Lubricants
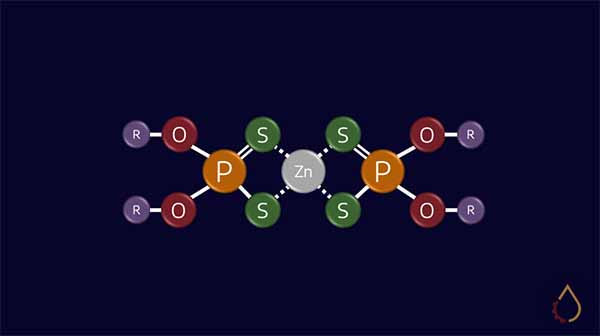
ZDDP is an organo-metallic compound with four sulphur atoms linked to a zinc atom. It is formed through the neutralisation reaction of zinc oxide and thiophosphoric acids. Although it is impossible to image the molecule, the structure has been determined to look something like the following:
ZDDPs protect metallic surfaces by building sacrificial layers on metal surfaces composed of glassy iron, zinc polyphosphate, and metal sulphides. Under mixed lubrication conditions, when contact between surface asperities is occasional, ZDDP reacts with just the asperities to reduce contacts. At loads that cause the oil film to collapse, ZDDP reacts with the metal surfaces to prevent friction welding.
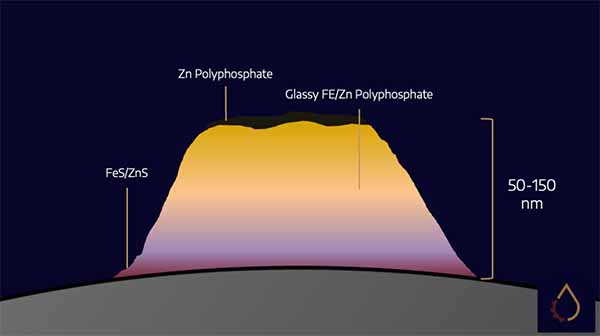
ZDDPs form these “tribofilms” by decomposing under high temperature/high-pressure conditions. This is a highly complex process that varies depending on the temperature, pressure, and exact species of ZDDP in use – formulators can use this variation to adjust the characteristics of the tribofilm formed. Analyses have shown that the thickness of this film can be as low as 20 nm.
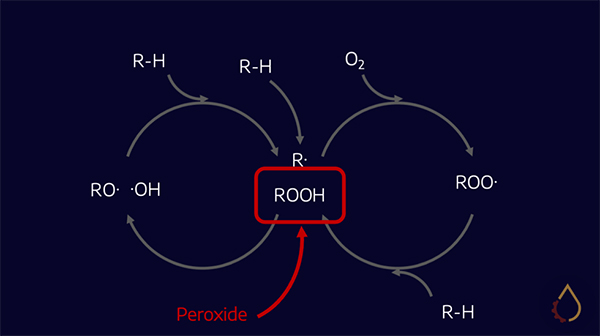
The party trick of the ZDDP family is their capacity to also function as effective antioxidants. When base oils degrade, they form hydroperoxides as part of the “auto-oxidation cycle”, but ZDDPs intercept and decompose them into inactive products. This has the secondary benefit of reducing the corrosion caused by high concentrations of hydroperoxides.
Declining Zinc Levels in Engine Oils
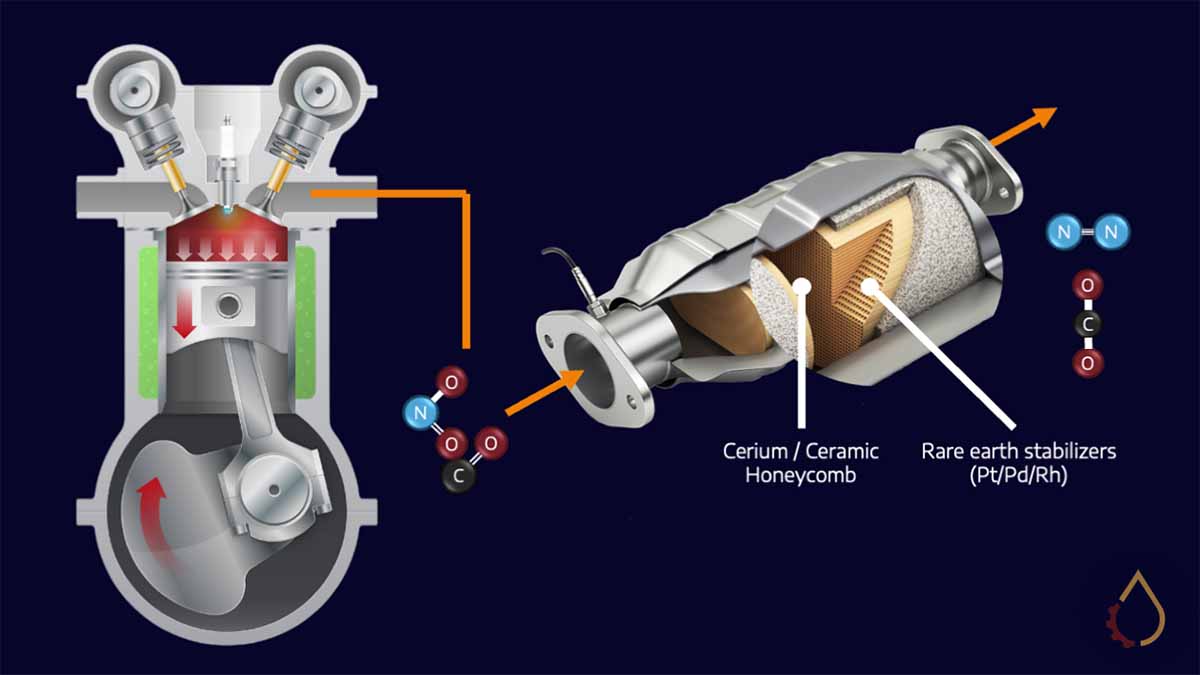
If you take a sample of engine oil from the mid-20th century engine oils, you would expect to measure 1400-1600 ppm zinc. The combination of low price point, engine protection and lubricant longevity made a high dosage of ZDDP inevitable. That all changed with the USA Clean Air Act in 1975, which required a 75% decrease in emissions in all new model vehicles. Such a drastic decrease was only possible with the use of catalytic converters.
Petrol and diesel combustion causes the release of undesirable compounds such as carbon monoxide, nitrogen oxides and soot. Catalytic converters are an effective way to transform these into relatively harmless carbon dioxide and nitrogen. Still, the process requires rare earth metals in the converter to be exposed to exhaust gases.
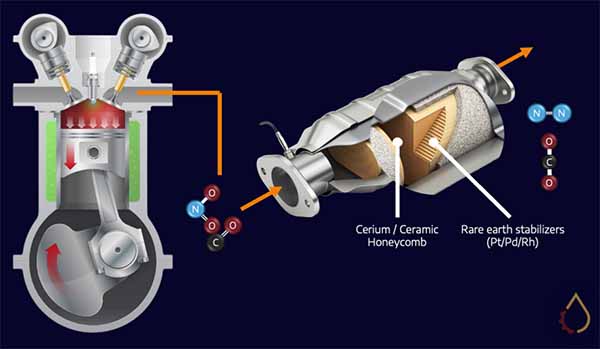
The exact property of ZDDPs that make them ideal antiwear components (affinity for metals and formation of glassy films at high temperatures) makes them a nightmare for these converters. In particular, the formation of zinc pyrophosphate irreversibly deactivates catalyst metals. This has necessitated a reduction in the ZDDP treat rate for engine oils, with new ACEA and API categories specifically limiting phosphorous levels in the formulations.
With Zinc levels declining, the automotive industry has two choices: find alternative molecules to replace ZDDP’s performance or make antiwear additive redundant through improved materials. Currently, the industry is engaging both solutions; boron and molybdenum additives are being used in increasing quantities, as are diamond-like coatings (DLCs) in areas such as the valve train. Using DLCs in combination with softer springs helps reduce the need for additives; for asset managers and reliability engineers, it reduces the ability to monitor valve train wear because pure carbon is not included in ICP test results.
Although some other technologies, such as ionic liquids, present promise, zinc is unlikely to disappear from engine oils anytime soon. Instead, a slow reduction is likely in the coming decades.
No-Zinc Gear Oil Formulation
In the 1960s, there was little to distinguish industrial lubricants from engine oils. For the most part, industrial applications like enclosed gears used cut-back, automotive-style formulations.
It wasn’t until the 1970’s that the industry began to recognize that industrial drain oil cycles are significantly longer than automotive. A typical engine oil might stay in the application for 2000 hours before the oil is drained, but industrial lubricants may last a year or even longer. This largely eliminated metal-containing additives from formulations because they act as oxidation catalysts, and their polar nature attracts water.
However, there are still some legacies that have been passed down from the mid-20th century. The Caterpillar TO-4 specification, for example, requires a minimum quantity of 900 ppm zinc, and the last generation of wind turbine gear oils sometimes contained high levels of metals. This presents challenges for asset managers because the operating philosophies of these lubricants are opposed to one another.

Engine oils are designed to absorb contaminants such as combustion products, partially oxidized hydrocarbons, and reduced hydrocarbons. These enter the crankcase via blow-by past the piston rings, where they are mixed with the engine oil. Engine oil additive systems are designed to capture, chelate, or dissolve the contaminant until the lubricant flows to the filter allowing removal, or held in suspension until the oil is drained. The polar metal additives contained in these oils are hygroscopic and tend to absorb water. Lubricants with their heritage in engine oils should therefore be treated with a high degree of filtration. In high water environments, all efforts are taken to minimize water ingress into the application, less it is carried directly to the load zone by the additive package.
In the non-metal formulating tradition, the lubricant is designed so that the contaminants settle out. Less filtration may be required, but this necessitates adequately sized reservoirs for sufficient settling time. The demulsibility characteristic of the oil is crucial to enable the water to separate from these systems, so trending of this parameter through used oil analysis should be exercised.
Low Zinc Hydraulic Oils
Zinc has long been a feature of hydraulic oils, with demanding OEM tests such as the Bosch Rexroth RE 90220 placing a high value on antiwear performance. Historically formulators have achieved good protection of swash plates and pump vanes using high zinc treat rates, but this is beginning to fall out of favor as the industry shifts to higher-efficiency hydraulic packs.
This is particularly acute in the mobile hydraulics market, where weight savings equal fuel efficiency. Units are being designed comparatively smaller than previous iterations while maintaining or exceeding previous power output. The result is more power density and, therefore, more heat generation. Increasingly, oil temperatures over 90oC are being observed, and this is approximately the point at which ZDDP begins to degrade thermally, resulting in increased sludge formation. This sludge can then impact the operation of hydraulic packs by restricting flow past servo valves causing a “sticky” function. The reduced thermal efficiency of oil coolers resulting from sludge coating the surface area accelerates the process.
The implication for asset managers is to understand the impacts for asset condition monitoring.
Zinc-based hydraulic oils operating at high temperatures add two nuances; the first is to add varnish detection testing methods to the standard used oil analysis slate, as these can pre-empt operational issues. The second is to understand how to track additive depletion because the sludge in hydraulic systems running on ZDDP-laden oils typically contains zinc but not phosphorus. This results from zinc dissociation out of the rest of the molecule at high temperatures – but the sulphur and phosphorous, which remain, continue to act as potent antiwear additives.
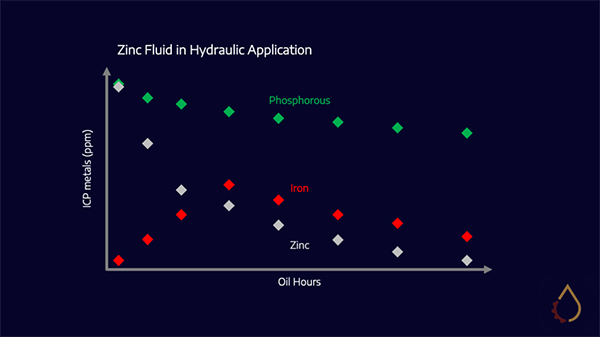
It is essential to understand the alternative antiwear technology employed for zinc-free systems. Is it MoDTC, MoDDP, an ash-free phosphorous or nitrogen-based polyamines? Nitrogen-based additives are complicated to trend as Nitrogen is not reported as part of an ICP panel. Therefore the presence of wear metals in the oil is a better indicator of wear performance.
The Future of ZDDP
Zinc still has a place in future oil formulations but combining regulation and emerging technologies diminishes its role. For end-users, the key is to understand the impact of the transition on lubricant performance, equipment life and condition monitoring strategies.