Just as there are various states of matter, there are different lubricant types. The four main categories are gas, liquid, cohesive (grease), and solids. In the industrial lubrication world, we deal with fluids and greases, but some operating environments make these unsuitable – in these circumstances, we often turn to solid lubricants.
The Lubrication Mechanism of Solid Lubricants
With oils and greases, we typically rely on the formation of a fluid film to separate two surfaces in relative motion. When loads are too high or speed too low, we enter the mixed and boundary regimes and rely on additives such as antiwear and EP agents to protect machine surfaces.
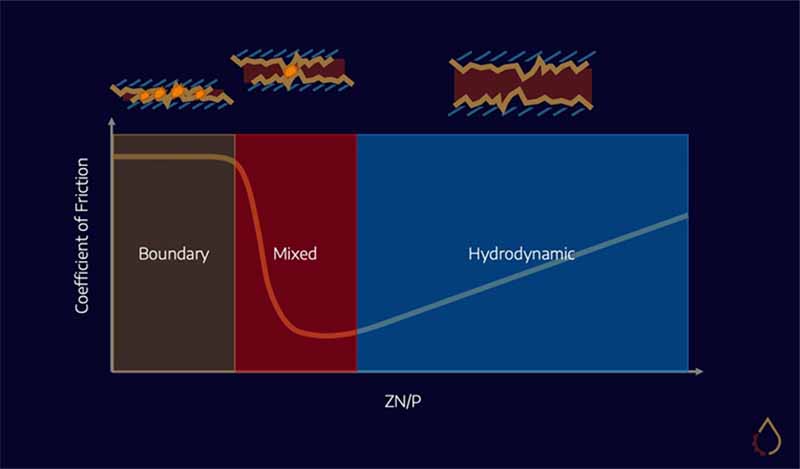
Solid lubricants act exclusively in the boundary regime. Their lubrication mechanism is based on their ability to form a thin film on the surfaces of moving components. This film (sometimes known as a “tribofilm”) acts as a barrier between the surfaces, helping to reduce adhesive wear. These particles can also reduce the friction between the surfaces thanks to their very low shear strength.
Structure of Solid Lubricants
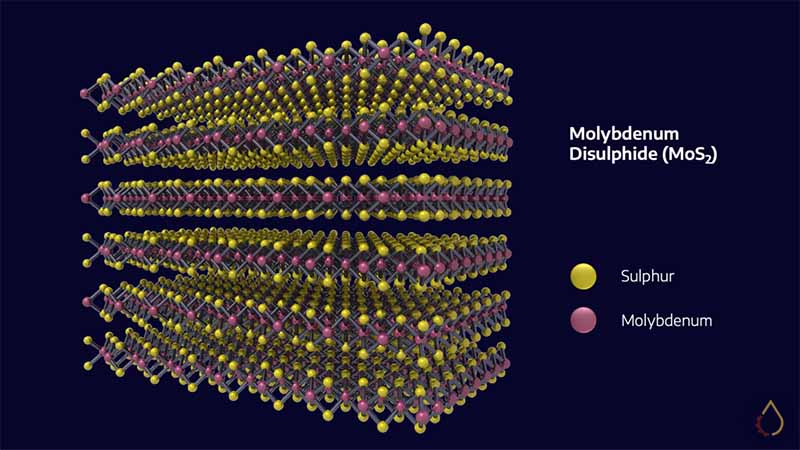
Understanding the friction-reducing properties of solid lubricants helps to understand their underlying structure. Most solid lubricants are lamellar solid materials consisting of atomically-thin planes. That’s a bit of a word salad, so above is an image that will give you a better idea of what that means.
The individual layers (in the case of MoS2, three atoms thick) are held together only by weak Van der Waals forces and are easily sheared by relative motion. This action is like a person running on top of a floor covered in playing cards.
The thickness of the tribofilm is a crucial factor in the lubrication process. A thin film provides a low coefficient of friction and reduces wear, while a film that is too thick can cause an increase in friction and wear.

The thickness of the tribofilm can be influenced by several factors, including the type of solid lubricant used, the load on the moving parts, and the environment in which the lubrication is taking place.
Solid Lubricant Selection
There are several common choices when selecting a solid lubricant or grease containing solid lubricant. It’s helpful to understand the properties of each and their strength and weaknesses:
Graphite
Graphite is a naturally occurring form of carbon. It is a good lubricant for high-temperature applications and has a low coefficient of friction. It operates like the low friction contact between your graphite pencil and paper, as single layers of graphite (technically now known as “graphene”) shear off.
Graphite is unusual in that it requires environmental gases to form a tribofilm. As Dr. Juan Preciado Flores discussed in Episode 9 of the Lubrication Experts podcast, “carbon needs a passive layer to have a lower coefficient of friction.
That’s why for a pin-on-disk test in a dry environment, you may have a CoF (coefficient of friction) of 0.4. And if you add a little bit of water or increase the room’s humidity, you may have a coefficient of friction of 0.2 because you are helping that tribofilm by adding a reactive gas.”
It is also highly conductive, which may benefit slip-ring contact applications but can be detrimental elsewhere.
Molybdenum Disulfide (MoS2)
MoS2 (commonly just referred to as “moly”) is a compound that is a good lubricant for high-temperature and high-pressure applications and has a low coefficient of friction. It is mined from some sulphide-rich deposits, then refined to achieve a purity suitable for lubricants.
The high-temperature performance of MoS2 is limited to 400 °C, a limit at which the molecule experiences excessive oxidation. Water has the reverse effect on MoS2 compared with graphite, as it often reacts with the molecules, increasing the shear strength between the layers and therefore increasing the coefficient of friction.
Boron Nitride (BN)
BN is a compound made of boron and nitrogen and is an excellent lubricant for high-temperature and high-pressure applications. It has a low coefficient of friction and is highly resistant to thermal shock. BN is often used in applications such as furnace door lubrication and in high-temperature chemical environments where MoS2 isn’t an option.
There are numerous forms of Boron Nitride (known as “polytypes”). Hexagonal boron nitride (h-BN) has a layered structure like graphite, whereas cubic boron nitride (c-BN) is analogous to diamond and used in abrasion applications.
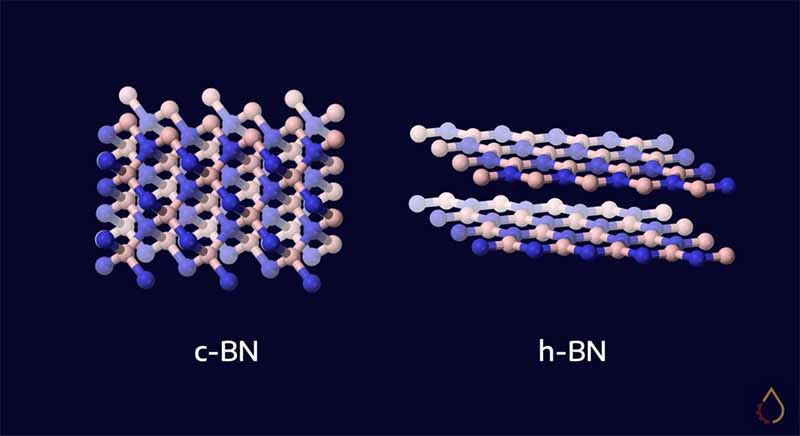
Within each layer of h-BN, boron and nitrogen atoms are bound by strong covalent bonds, but weak van der Waals forces mean the layers aren’t firmly bound to each other. This gives it a low shear strength, typical of other solid lubricants.
Fluoropolymers (e.g., PTFE)
Fluoropolymers (usually PTFE in lubricants) are the exception to the rule. Rather than having low-shear layers, the molecules of PTFE slip past each other very easily because the outer fluorine atoms are so tightly-packed. PTFE can typically be used up to a temperature limit of 260°C and has good resistance to reactive chemicals.
Applications of Solid Lubricants
Un-mixed solid lubricants have a wide range of uses in industries where liquid lubricants and greases cannot be used. The space industry has long relied on dry lubricant coatings for spacecraft, satellites, and lunar/Mars landers as liquid lubricants would boil in the extreme space environment. Application methods vary, but the solid lubricants are frequently ionized and sputtered onto the equipment’s surface.
We commonly see solid lubricants included in greases as an additive in an industrial environment. This is especially true in high-load, low-speed applications typical to the mining and construction industries, where greased gears, bushings, and even rolling element bearings cannot establish a fluid film.
They provide excellent wear protection and can withstand harsh operating conditions in these industries. In some high-washdown scenarios, we may rely on the residual solid lubricant that survives water blasting to lubricate a bucket pin before the element can be re-greased.
You may have also encountered the use of Moly and Boron in liquid lubricants. These are typically present in soluble forms like Molybdenum Dithiocarbamate or Borated Esters and work as multifunctional additives to provide some antiwear protection and anti-oxidancy.
Soluble molybdenum compounds provide their antiwear function by decomposing into MoS2, which then adheres to the load surface and functions the same way a solid lubricant would.
The Future
R&D into solid lubricant technologies continues to advance, with engineers and scientists developing solid lubricants that can withstand more severe conditions and offer better wear protection.
There are some exciting developments in nanocomposite solid lubricants. The lubricants can be made of nanoparticles from different types of solid oils (such as MoS2 combined with graphite in alternating layers). Combining the best properties of each molecule can achieve both high-temperature and high-pressure lubrication.
They may also benefit from the sustainable transition; as more businesses look to reduce their use of fossil fuels, machine surfaces impregnated with solid lubricants or solid lubricants replacing liquid lubricants may become more common.