Typically, when an oil undergoes degradation, the first culprit to be blamed is oxidation. We often hear that the oil has oxidized, producing varnish, leading to its degradation. While this simple statement may seem plausible, it is not the only way oil can degrade.
If an oil has undergone oxidation, the real question we should be asking is not how much varnish has been produced but what caused the oxidation in the first place?
In this article, we will explore the various ways in which an oil can degrade via oxidation. However, as you know from previous articles, other degradation methods exist.
How Can Oxidation Occur?
Before diving further into the root cause of oxidation, one must first fully understand how oxidation occurs. When truly investigating a root cause for a failure, we should start with the question “How could?” rather than “Why?”.
This line of questioning heavily influences the answers. The “How could?” responses stem from a more evidence-based approach.
On the contrary, if we question “Why?” this is more opinionated and can mislead the investigation towards a biased opinion rather than the facts.
This leads us to the main question, “How can oxidation occur?”.
According to Ameye, Wooton, and Livingstone, 2015, oxidation occurs when there is any reaction in which electrons are transferred from one molecule. Ideally, in oxidation, during the initiation phase, free radicals are formed, which in turn produce more free radicals.
A free radical is a molecular fragment with one or more unpaired electrons which are accessible and can easily react with other hydrocarbons, as explained by Ameye, Wooton, and Livingstone, 2015.
After the initiation phase, which has the free radicals, the propagation phase follows, in which the antioxidants react with these free radicals to make them more stable. This is part of the reaction in which there is usually a drastic depletion of antioxidants or where the oil becomes sacrificial.
The antioxidants act as a barrier to protect the base oil from oxidizing. However, they can no longer protect the base oil once they become depleted. This leads to the termination phase, where the remaining free radicals attack the base oil.
As a result, this gives rise to the condensation phase, where we begin to physically notice the changes in the oil’s viscosity and the presence of insoluble by-products. These are the deposits that are known are lube oil varnish to some but can further be defined by their chemical composition.
Understanding how oxidation occurs can assist us in determining the root cause when an oil degrades. It allows us to identify the different stages to further help us determine if it is indeed oxidation that is occurring or not.
What Evidence Is Needed?
However, understanding the oxidation process is just one part of the puzzle. When performing an investigation, we also need to know what factors or characteristics should be present. Additionally, we need to prove that their presence confirms our hypothesis of whether or not oxidation is occurring. This is where the line of evidence-based questioning plays a significant role.
When oxidation occurs, it is usual to see the presence of aldehydes, ketones, hydroperoxides, and even carboxylic acids. These can be confirmed using the FTIR (Fourier Transform Infrared test).
Typically, one will also find some deposits in the system. These deposits can be further characterized and tested to determine their nature using FTIR. Their presence, however, may be confirmed using the MPC (Membrane Path Colorimetry, ASTMD7843) test.
Identifying the presence of the deposits and/or the compounds listed above can lead to the conclusion that oxidation has occurred.
Another critical characteristic of oxidation is the depletion of antioxidants. This can be easily identified by utilizing the RULER® (Remaining Useful Life Evaluation Routine) test. This test quantifies the remaining antioxidants in the oil and gives the value for the amines and phenols (which is very important, especially in synergistic mixtures).
As such, one can detect the trend in the depletion of antioxidants and implement measures to prevent this before they become depleted.
The main tests to assist in determining the presence of Oxidation include:
RULER (Remaining Useful Life Evaluation Routine) levels less than 25% compared to new oil. This value represents the level of antioxidants in the oil. Hence, low levels indicate that the antioxidants are decreasing, possibly due to oxidation. This test can accurately give information on whether oxidation is currently occurring in the oil before deposits are formed.
An increase in acid number indicates the presence of acids resulting from oxidation. However, it must be noted that this change in acid number only occurs after oxidation has taken place. Hence this test is not a good indicator to determine if oxidation is occurring; instead, it is more definitive in letting us know that oxidation has already occurred.
Rapid color changes – darkening of the oil due to the deposits being present. While color is not the best indicator, in some instances, the darkening of the oil can provide a bystander to ask whether something is occurring in the oil. It is not a definitive test for the presence of oxidation.
FTIR test (Fourier Transform Infrared) for the presence of insolubles formed during the oxidation reaction. This can accurately determine the presence of any compound to assist us in determining whether oxidation is occurring.
MPC (Membrane Patch Colorimetry) levels outside the normal range (above 20). This lets us know that insoluble deposits are present in the oil. One must note that there may be instances where the deposits might not appear in the MPC test. As such, this should not be a standalone test to determine the presence of deposits.
RPVOT (Rotating Pressure Vessel Oxidation Test) levels are less than 25% compared to new oil (this is the warning limit). This is the industry standard, but this test does not have a high repeatability value in that if the same test were performed on identical samples, the values would be different. Additionally, the value (reported in minutes) is not easily translated into the environment of the components.
These tests provide us with the evidence we need to determine the presence of oxidation when performing the root cause analysis on the component’s failure.
Determining the Root Causes
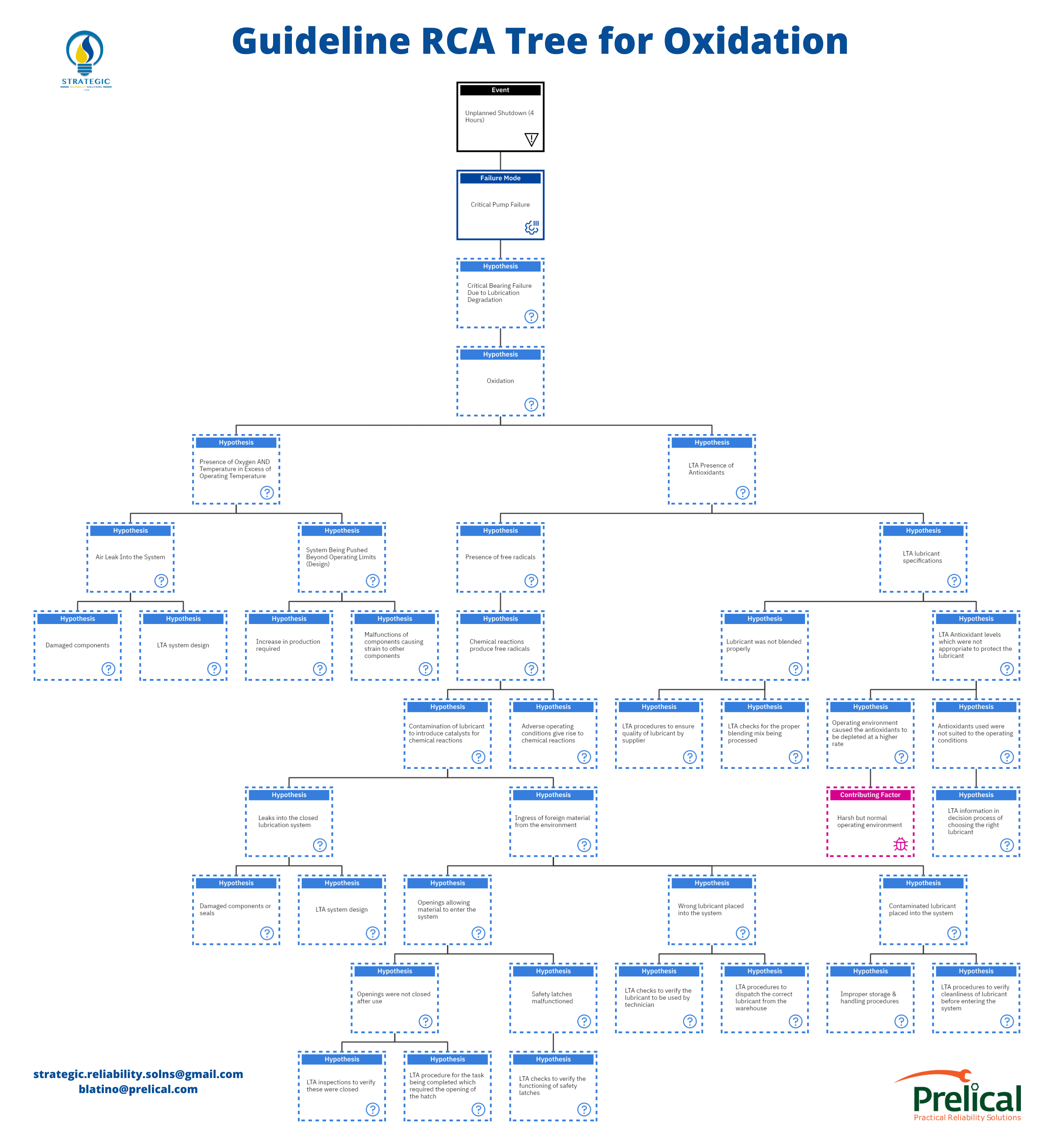
Finally, we’ve arrived at the point where we can effectively determine the root cause. It is critical that the analyst understands oxidation and has knowledge of the evidence needed before embarking on the root cause journey. As noted in the first part of this article, the question we should ask is, “How could?”.
We hypothesize that oxidation is occurring. In a complete root cause analysis, we should hypothesize the occurrence of all the degradation mechanisms and eliminate them with evidence-based data.
There are two main ways in which oxidation can occur either through the presence of oxygen and temperature over the normal operating temperature of the system or if there is a less-than-adequate presence of antioxidants.
If we follow our line of questioning with the presence of oxygen and temperature and again ask, “How could?” we can get two primary responses. Either there was an air leak in the system, or the system was being pushed beyond its operating limits.
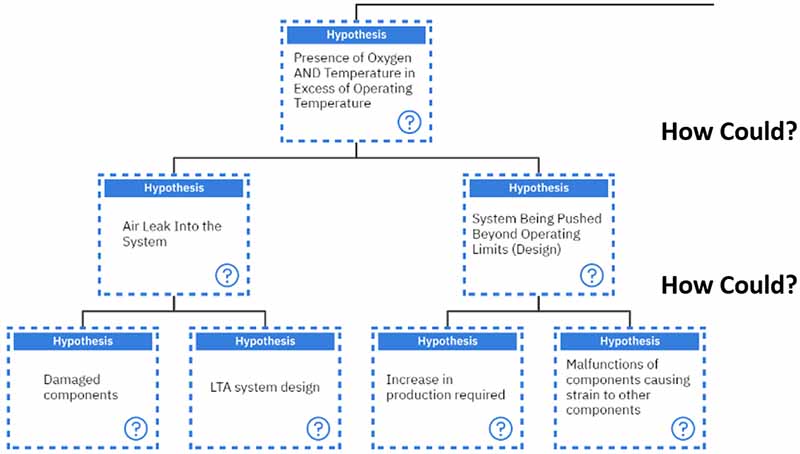
If we further investigate the air leak into the system, we ask, “How could it?” again. There are two main ways: either there are damaged components, or a less-than-adequate system design allows air to enter the system.
If we follow the pathway of investigating “how could” the system be pushed beyond its operating limits, then we can come up with two hypotheses. Either an increase in production was required, or there was a malfunction of the components, which caused strain on the other components.
Both of these hypotheses are physical and can be investigated further, but we will focus on the lubricant aspect of this article. Hence, we will follow the questioning surrounding the less-than-adequate presence of antioxidants.
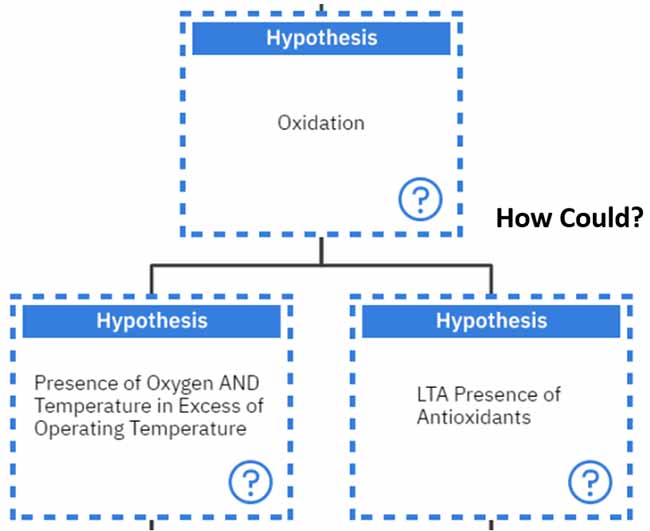
We begin with the question, “How could we have a less-than-adequate presence of antioxidants?”. From the information gathered in this article, we know this can result from free radicals or less than adequate lubricant specifications.
We will investigate the “Presence of free radicals” first.
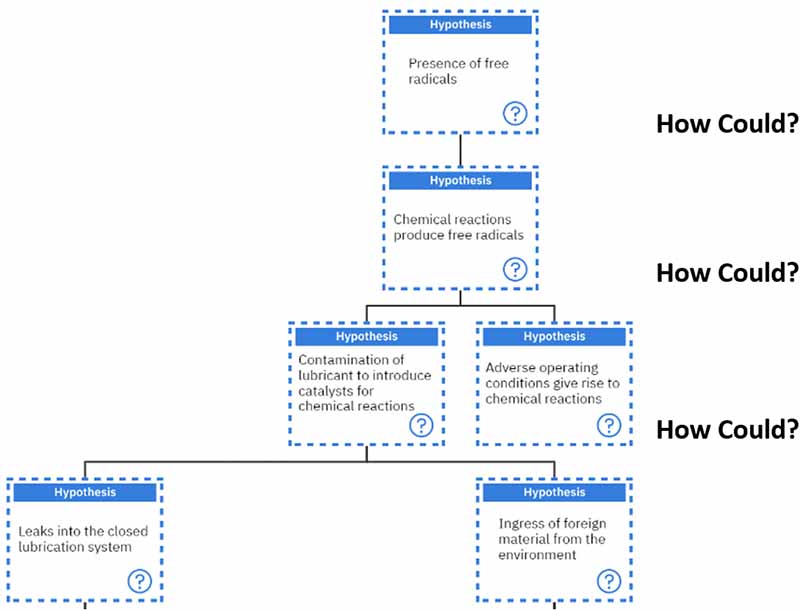
“How could we have the presence of free radicals?” Free radicals can emerge as a result of chemical reactions.
“How could these chemical reactions produce free radicals?” There are two main ways in which this can occur. Either the lubricant got contaminated, which introduced catalysts for these chemical reactions, or adverse operating conditions gave rise to these chemical reactions.
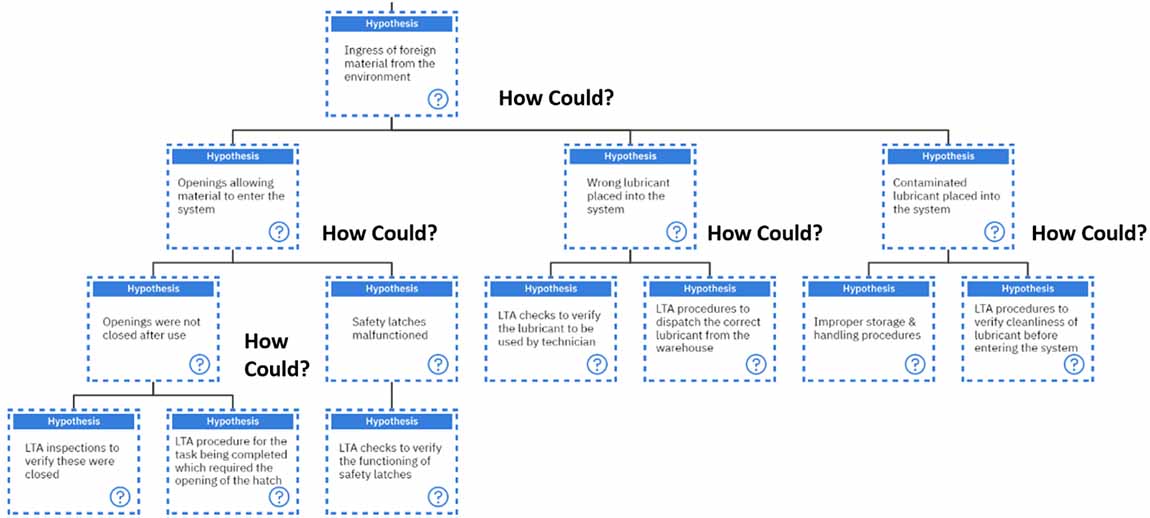
Then, we must ask again, “How could we have contamination?” Contamination can occur if leaks are getting into a closed lubrication system or if there is ingress of foreign material from the environment.
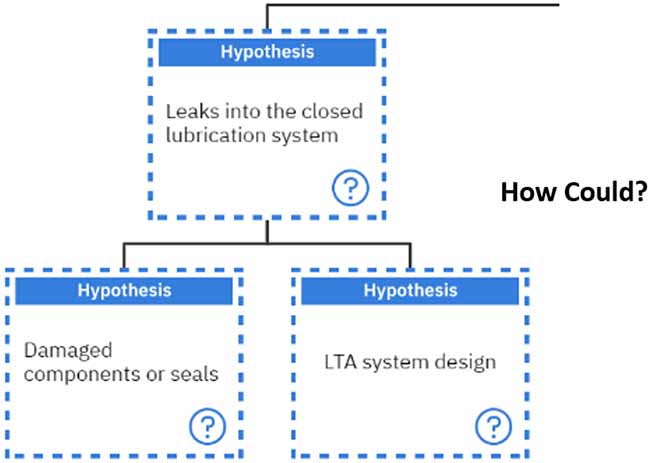
Our line of questioning continues when we ask, “How could we have leaks in a closed lubrication system?”. These can result from damaged components or seals allowing leaks into the system or if the system is less than adequately designed.
These are physical attributes of the system, so we will go back to investigating the lubricant aspect.
This is where we get to ask our famous question, “How could we have ingress of foreign material from the environment?”. Ideally, this can be classified in three ways;
- There are openings which are allowing materials to enter the system or
- Wrong lubricant was placed in the system or
- Contaminated lubricant was placed in the system
Let’s investigate all three aspects, starting with the openings allowing foreign material to enter the system. There are two main ways in which this can occur. Either the openings were not closed after use, or the safety latches malfunctioned.
Suppose the openings were not closed after use. In that case, there is a possibility that there were less than adequate inspections to verify that these were closed after use or a less than adequate procedure for the task being completed which required the opening of the hatch.
On the other hand, if the safety latches malfunctioned, this could result from less than adequate checks to verify the functioning of the safety latches.
In these cases, the root causes are not the physical elements but rather the systemic reasons for these procedures not being adequately performed.
Now we investigate the second central hypothesis, “How could the wrong lubricant be placed in the system?” While there are many ways in which this can occur, we have narrowed it down to two main areas.
Either there were less than adequate checks to verify that the technician received the correct lubricant, or there were less than adequate procedures to dispatch the correct lubricant from the warehouse. We will not go further into these two as they are now systemic causes that must be addressed.
Onto the third hypothesis of “How could a contaminated lubricant be placed in the system?”. There are two main avenues for this to occur. Either there were improper storage and handling procedures, or there needed to be more adequate procedures to verify the cleanliness of the lubricant before entering the system.
The other hypothesis stemming from the “less than the adequate presence of antioxidants” is having “less than adequate lubricant specifications.” Let’s investigate this one a bit further.
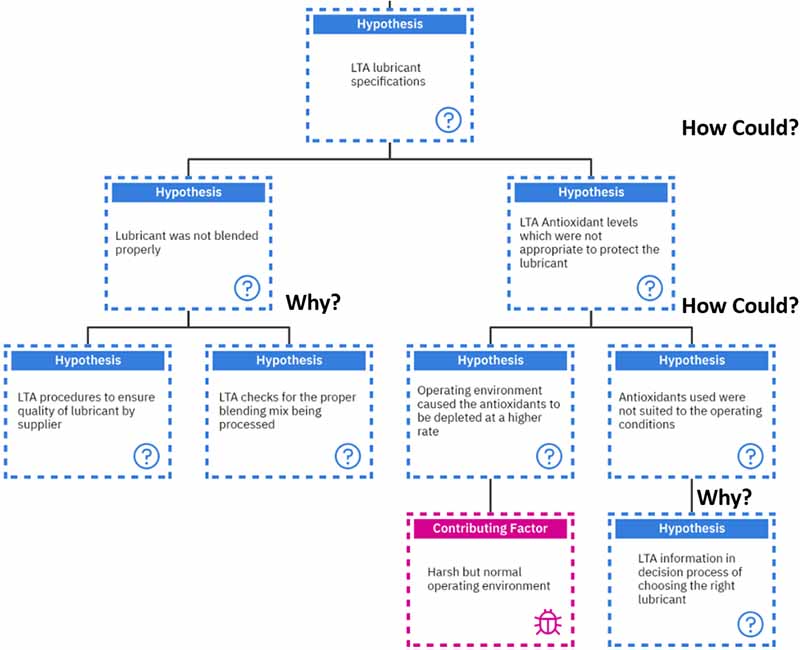
“How could we have a less than adequate lubricant specification?” Typically, this can result from the lubricant not being blended properly or less than adequate antioxidant levels, which were inappropriate to protect the lubricant.
Now, the line of questioning changes to “Why?” as we have gone past the physical element and some decision-making was involved in this hypothesis. We must ask, “Why wasn’t the lubricant blended properly?”
This can result from less than adequate procedures to ensure the quality of the lubricant by the supplier or less than sufficient checks for the proper blending mix being processed.
These are factors one should consider when receiving any lubricant from their supplier.
On the other hand, if we follow the line of questioning of “How could there be a less than adequate antioxidant level to protect the lubricant?” we can come up with the following.
Either the operating environment caused the antioxidants to be depleted at a higher rate. This would be as a result of a harsh but normal operating environment. In this case, we may be unable to make those environmental changes (without the OEM’s consent).
Or the antioxidants used were not suited to the operating conditions. This is where the line of questioning again shifts to “Why were they not suited?”. This could result from inadequate information in choosing the right lubricant suited for the system.
What Is the Real Root Cause of Oxidation?
From the logic tree that we have created, we can see that there is no sole root cause for oxidation. It can stem from various causes, including physical, human, and even systemic roots. The main takeaway from this exercise is to acknowledge that root causes are not limited to physical causes, such as leaks in the system.
Instead, the actual root causes can be linked to systemic areas of concern where there may not have been enough information to guide the analyst in choosing the most ideally suited lubricant for the application. There are also root causes related to the lubricant not being appropriately blended.
It is critical to thoroughly investigate the real root causes when the lubricant becomes degraded to avoid being stuck in the loop of constantly experiencing degradation.
For more info on other methods, check out the book Bob Latino, and I authored called “Lubrication Degradation – Getting Into the Root Causes,” published by CRC Press.
References:
Ameye, Jo, Dave Wooton, and Greg Livingstone. 2015. Antioxidant Monitoring as Part of a Lubricant Diagnostics – A Luxury or Necessity. Rosenheim, Germany. February 2015.
Latino, Bob, Sanya Mathura. 2021. Lubrication Degradation – Getting into the Root Causes. CRC Press, Taylor & Francis.