Lubricating oils play a critical role in rotating machinery’s efficient and safe operation. However, these fluids are exposed to various factors that can deteriorate their performance over time. Two of the most common degradation mechanisms are oxidation and thermal degradation, each with distinct causes, effects, and failure modes.
This article explores the differences, from a chemical point of view, and the physical changes between the two processes, their consequences on machinery, and their early identification.
On many occasions, both terms are misused as synonyms when in reality, the processes are entirely different.
Oxidation: The Chemical Chain Reaction That Starts It All
Oxidation is a chemical process in which oil reacts with oxygen present in the air. The concentration of the air in the oil varies depending on variables such as flow rate, viscosity, or temperature. The percentage of air in oil, for example, in the return line of a gas turbine tank can be between 6 and 12%, while that same oil in the bearing area can have an air concentration of less than 2% [1]
Oil oxidation in the presence of oxygen is a complex chemical process that involves a series of chain reactions. Additionally, these reactions can be catalyzed by heat, pressure, metals, and contaminants.
Factors that influence oxidation:
- Temperature: At higher temperatures, the rate of reaction with oxygen increases exponentially.
- Presence of catalyst metals: Iron and copper wear particles can accelerate oxidation.
- Exposure time: The longer the oil is in contact with the air, the greater the oxidation.
- Working load: In gas turbines and engines, high load generates temperatures that can induce premature oxidation.
Oxidation follows a clearly defined process where the following stages are identified:
Initiation
The process begins with the formation of free radicals in the molecules of the base oil. This can occur for various reasons, including time, improper storage, and more aggressive factors in the machine.
Propagation
Free radicals react rapidly with molecular oxygen (O2) to form peroxide radicals (ROO•).
Degradation of Hydroperoxides
Hydroperoxides (ROOH) are unstable and break down thermally or catalytically into organic acids, aldehydes, and ketones that can be monitored by advanced analysis.
Formation of Insoluble Compounds
Over time, radicals and secondary oxidation products (such as aldehydes, ketones, and carboxylic acids) react with each other to form insoluble polymers, often called varnish and sludge. These reactions are known as cross-polymerization and result in high molecular weight compounds.
- Varnishes: Semi-solid and brittle compounds that adhere to metal surfaces.
- Sludge: Sticky rubber-like materials that clog filters and ducts.
Byproducts such as aldehydes, ketones, and carboxylic acids can be determined and quantified long before they impact the acid number (AN), as in many cases, this can be a late indicator of chemical reactions in the oil.
On the other hand, although it may seem contradictory, one of the indicators of lubricant oxidation is precisely the determination of the degree of oxidation by FTIR. In the oxidation analysis of lubricating oil by FTIR (Fourier Transform Infrared Spectroscopy), the degradation of the oil is mainly measured in the absorption region corresponding to the carbonyl groups (C=O), which appear when the oil is oxidized, region: ~1,710 cm⁻¹ (±10 cm⁻¹).
Other bands that may indicate secondary oxidation:
3,400 cm⁻¹ → Hydroxyl (-OH) groups of advanced oxidation products.
1,230 – 1,150 cm⁻¹ → Oxidized esters and additive degradation products.
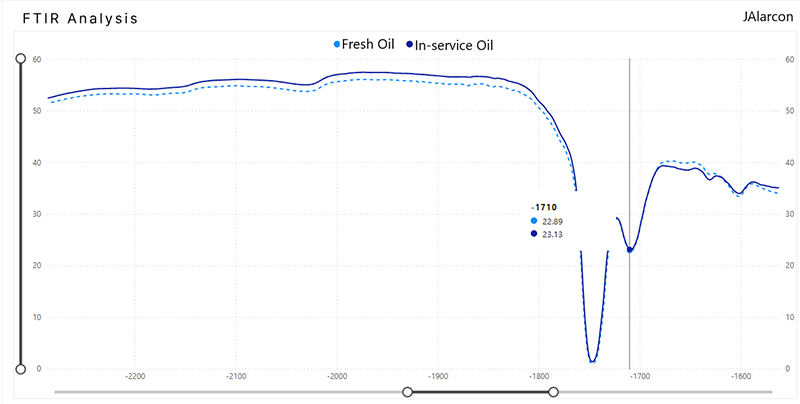
The figure above shows that the variation in the oxidation of turbine oil is minimal after having been in service for about 12000 hours, with a variation of less than 3% in oxidation.
Thermal Degradation: When Heat Breaks Oil at the Molecular Level
Unlike oxidation, thermal degradation is when base oil molecules and additives are chemically broken down or transformed due to high temperatures or constant exposure to medium-high temperatures without oxygen. This happens when temperatures exceed the thermal stability of the lubricant, causing the breakdown of molecular chains and the formation of undesirable byproducts.
Factors that influence thermal degradation:
- Temperature spikes: Contact with surfaces that are at a high temperature (such as bearings).
- Extreme workload: Natural gas engines and turbines operating at full capacity for an extended period of time generate excessive heat.
- Oil residence time: The longer the oil is exposed to extreme heat without proper cooling, the greater the degradation.
Chemical Mechanisms of Thermal Degradation
At high temperatures, the chemical bonds in the base oil molecules can break; this phenomenon is known as cracking, which leads to two factors in parallel.
Homolytic rupture
C−C or C−H bonds break down by generating free radicals:
![]()
Additive breakdown
Certain additives such as antioxidants, antifoam, and dispersants, among others, have thermal limits. At extreme temperatures, additives degrade, forming inactive compounds or even secondary compounds that contribute to the formation of deposits.
Thermo-oxidation
Also, at high temperatures, a phenomenon called Thermo-oxidation. Although oxidation usually requires oxygen, at very high or prolonged temperatures, oxidation can occur without the need for atmospheric oxygen due to the internal breakdown of hydroperoxides (ROOH):
![]()

The figure above shows that the variation in the oxidation of turbine oil is about 7% after having been in service for about 2500 hours. However, due to a hot spot effect on the turbine, the ester-based defoamer additive drops drastically below 30% of the initial concentration, causing a Microdieseling problem in the oil.
Oxidation Failure Modes
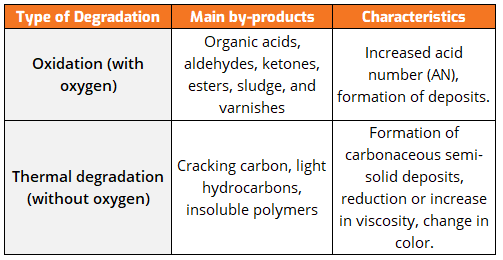

One of the primary debates in both cases is oil filtration. Inevitable degradation byproducts can be removed using filtration technologies. However, can the source of the byproduct generation problem be eliminated? In many cases where thermal degradation is the primary failure mode, a filtration system will systematically remove internally generated byproducts, but will it solve the root cause of the problem?
[1] JAlarcon Turbine Oil Analysis 2024