Antioxidants are additives that increase the oxidative resistance of the base oil to prolong lubricant life. They help prevent or reduce lubricant oxidation and work collaboratively with other additives in finished lubricants.
Understanding Oxidation: The Basis for Antioxidant Use
When speaking about antioxidants, the first thing that comes to mind is oxidation. This is the primary reason that antioxidants exist: to reduce oxidation. But what is oxidation, and why should there be antioxidants?
Oxidation occurs in everything in life (not just finished lubricants). We see oxidation regularly when we leave certain fruits exposed to the atmosphere (think about cut pears or apples). After being in the elements for some time, they are no longer fresh and have degraded slightly.
A similar reaction occurs during the oxidation of finished lubricants. Greg Livingstone provides an excellent summary of the oxidation process in his article, “Varnish, Deposits in Bearings, Causes, Consequences, and Cures.” The oxidation degradation pathway begins with initiation, where free radicals are formed in the presence of heat, wear metals, water, and oxygen as shown in Figure 1.
Afterward, during propagation, the free radicals form hydroperoxides, which can create oxidation by-products (Alkoxy radicals), eventually leading to high molecular weight oxygenated by-products.
During this process, the free radicals can also react with primary antioxidants, or the hydroperoxides can react with secondary antioxidants to slow these reactions. However, they will still form the high molecular weight oxygenated by-products once depleted.
Next in the termination phase is polymerization and agglomeration, followed by the physical and chemical changes to the lubricant. It must be noted that there are various stages to oxidation, and typically, when we see sludge or varnish, oxidation has already occurred.
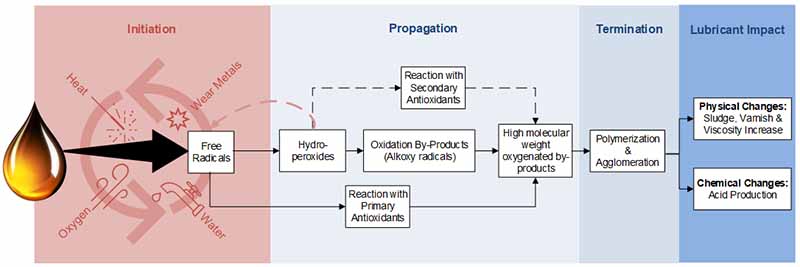
Figure 1: Summary of the oxidation process.
When oxidation occurs, the oil quickly loses its antioxidants; they can no longer protect the oil. As such, the oil begins to undergo physical changes where sludge and varnish appear, and viscosity usually increases. These oils also experience a rise in acid production after these reactions occur.
Now that we have a better understanding of oxidation, whereby the antioxidants are deployed to help reduce the oxidation rate, we can dive deeper into the world of antioxidants and how they can help fight against oxidation for the finished lubricant.
How Antioxidants Combat Oxidation in Lubricants
As the name suggests, antioxidants prevent oxidation; thus, it is no surprise that they are also called Oxidation inhibitors. During the refining of the base oil, the natural antioxidants are typically stripped away.
Thus, additional antioxidants must be added to the finished lubricant to ensure it does not oxidize as quickly. It is important to note that antioxidants can reduce the amount of oxidation that occurs but will not stop it completely.
Some of the natural antioxidants in base oils include polycyclic aromatics and sulphur and nitrogen heterocyclics6.
During oxidation, acids, and peroxides are typically produced. Antioxidants added to finished lubricants usually contain hindered phenols and ZDDPs (zinc dialkyldithiophosphates). These will suppress the formation of the acids produced in these reactions.
Dedicated antioxidants are amines, phenols, and sometimes ZDDP, which also function as an antiwear additive. Antioxidants account for 3-7% of European and North American diesel and gasoline additive packages for finished lubricants6.
As mentioned earlier, antioxidants are not the only additives that have a role to play in protecting the equipment. Antioxidants often work together with detergents to help prevent corrosive wear, especially in engines.
Some moisture and acidic combustion by-products can enter the engine during oxidation and form acids. Detergents can help to reduce corrosive wear caused by these acids. Alkylphenols are often used as a substrate in the preparation of detergents; as such, they also exhibit some antioxidant properties.
It must also be noted that extreme pressure additives containing sulphur or phosphorus may also suppress oxidation. Contrarily, these additives decompose at very moderate temperatures, so their strength as an antioxidant is not generally promoted10.
Types of Antioxidants
Different additives have successfully suppressed the degradation of finished lubricants6, 10. These include:
- Radical scavengers/inhibitors, also called propagation inhibitors
- Hydroperoxide decomposers
- Metal deactivators
- Synergistic mixtures
Each of the above listed performs in a particular way to reduce the oxidation process in the lubricant.
Radical Scavengers
Many applications use radical scavengers as their preferred antioxidant. Radical scavengers are also known as primary antioxidants, as they are the first line of defense in the oxidation process. These are phenolic and aminic antioxidants.
They neutralize the peroxy radicals used during the initiation reaction to generate hydroperoxides4. These neutralized radicals form resonance-stabilized radicals, which are very unreactive and stop the propagation process.
Some examples of these additives are1: diarylamines, dihydroquinolines, and hindered phenols. While they may be known as simple hydrocarbons, they are often characterized by low volatility, used in quantities of 0.5-1% by weight, and have long lifetimes10.
These primary antioxidants are usually very effective at temperatures below 200°F (93°C)7. At these temperatures, oxidation occurs at a slower rate. These primary antioxidants are typically found in applications involving turbines, circulation, and hydraulic oils intended for extended service at these moderate temperatures.
Hydroperoxide Decomposers
The role of hydroperoxide decomposers is to neutralize the hydroperoxides used to accelerate oxidation. Radical scavengers (primary antioxidants) neutralize the free radicals, and these hydroperoxides are formed after the initiation stage. As such, hydroperoxide decomposers are known as secondary antioxidants.
These decomposers convert the hydroperoxides into non-radical products, which prevent the chain propagation reaction. ZDDP is one example of this type of decomposer, although organosulphur and organophosphorus additives have been used traditionally.
Metal Deactivators
Metal deactivators are usually derived from salicylic acid10. These function by entraining a metal such as copper or iron to inhibit oxidation acceleration. However, when the operating temperatures exceed 200°F (93°C), this is the stage at which the catalytic effects of metals begin to play a more important role in accelerating oxidation7.
Therefore, during these conditions, an antioxidant that can reduce the catalytic effect of the metals should be used, such as metal deactivators. These react with the surfaces of metals to form protective coatings. One such example is zinc dithiophosphate (ZnDTP), which can also act as a hydroperoxide decomposer at temperatures above 200°F (93°C).
Ethylenediaminetetraacetic acid is another commonly used example of this type of antioxidant.
Synergistic Mixtures
As explained above, there are different types of antioxidants, but one thing remains the same: They can work better when they work together. Some antioxidants can work in a synergistic manner to provide added protection to a finished lubricant. There are two types of synergism: homosynergism and heterosynergism.
Homosynergism occurs when two different types of antioxidants can be classed under the same category. One example is the use of two different peroxy radical scavengers. These both operate by the same stabilization mechanism but differ slightly in formulation.
On the other hand, heterosynergism is more common and often seen with aminic and phenolic antioxidants. Aminic antioxidants are primary antioxidants and radical scavengers, while phenolic antioxidants are secondary antioxidants and hydroperoxide decomposers.
In this case, the amnic antioxidants react faster than phenolic antioxidants, and as such, they are used up when the lubricant undergoes oxidation. However, the phenolic antioxidant regenerates a more effective aminic antioxidant, which helps suppress the rate of oxidation.
Testing Methods for Detecting Antioxidants in Lubricants
Since we now have more information about the various types of antioxidants and how they function to suppress oxidation, the next step is to determine whether they are indeed present in our finished lubricants.
The industry tries to identify the presence of antioxidants in a couple of ways. The first way is to measure the rate of oxidation, which does not give the exact value of remaining antioxidants. Instead, it gives the user an idea of how much oxidation has taken place based on other lubricant characteristics.
Then, the user must make an informed decision on the remaining life of the oil. On the other hand, there is one direct test to determine which antioxidants are present in the oil and provide their remaining quantity.
Some common tests in the industry that measure the oxidation rate include RPVOT (Rotating Pressure Vessel Oxidation Test), Oxidation via FTIR, Viscosity, and TOST (Turbine Oil Oxidation Stability Test).
While none of these actually quantify the remaining antioxidants in the oil, they all provide the user with an indication of the rate of oxidation currently occurring in the oil. We will dive deeper into these to understand how they assess oxidation rates.
RPVOT
In the industry, RPVOT has been used for decades to provide users with an idea of the rate of oxidation occurring in their oils. However, this test is performed where the sample is placed in a sealed container with pressurized pure oxygen and rotated at a high speed in a bath with a higher temperature to promote the oil’s oxidation8.
As oxidation occurs, there is a pressure drop in the vessel, and the rate of this pressure drop is compared to that of new oil. The final result is given in minutes. Typically, if the value falls below 25% of the original value, the oil is on its way out or almost at the end of its remaining useful life.
But what happens if the value is at 75%? Since the final result is given in minutes, it isn’t easy to correlate that value to a value in the field.
For instance, if the RPVOT result was 800 minutes, we cannot easily correlate that to a particular number of years or months of life remaining for the oil. Hence, this method does not truly measure the remaining antioxidants in the oil.
Oxidation via FTIR
Another way of measuring oxidation is by identifying its presence through FTIR (Fourier Transform Infrared) Spectroscopy. In this type of test, each element produces a unique fingerprint.
As such, oxidation produces a particular peak between 1600-1800 cm-1. There is no absolute reference for oxidation peaks; therefore, these are usually compared against the new oil samples9.
This test (ASTM D 7414) is usually used for engine oils rather than industrial oils. However, it still does not provide the user with the remaining antioxidants in the oil. Instead, only that oxidation has already occurred.
Viscosity
In the past, viscosity was usually cited as a method for detecting if oxidation was occurring in the oil. However, due to more recent discoveries and technological evolution, we have noted that the oil’s viscosity only increases after oxidation has occurred.
Therefore, it is not a valid test to identify if oxidation is happening in the oil, as there can be several reasons for the increase in viscosity. In the case of oxidation, the presence of varnish and sludge would account for this increase; however, this test still doesn’t indicate the remaining antioxidants in the oil9.
TOST
This test, developed in 1943, evaluates the oil after it is subjected to very specific conditions. Typically, the oil is stressed with high temperatures (203°F / 95°C), gross contamination (17% water), and substantial air entrainment in the presence of iron and copper catalysts5.
The oil’s life is measured by the time the sample takes to achieve an Acid number of 2 mg KOH/g.
As such, this test measures the amount of acid produced by an oil under extreme conditions. Again, it does not give us a quantifiable correlation to the oil’s actual field life. It must also be noted that this test is not suited for hydraulic or gear oils but is more tailored to steam turbine oils, which may undergo those simulated conditions.
RULER® test
The RULER test utilizes linear sweep voltammetry to detect the quantity of antioxidants remaining in the oil. It produces a graph showing peaks at the detected antioxidants. Typically, the used oil results are compared against the baseline data to determine the quantity of antioxidants in the oil (Fluitec, 2022).
This method allows users to specifically quantify and trend the decline of antioxidants in the oil over a period of time, as seen in Figure 2. This is very valuable as users can now provide a better estimate of the remaining useful life based on the trend of the decline of antioxidants, and by extension, this helps them to understand the health of their oil.
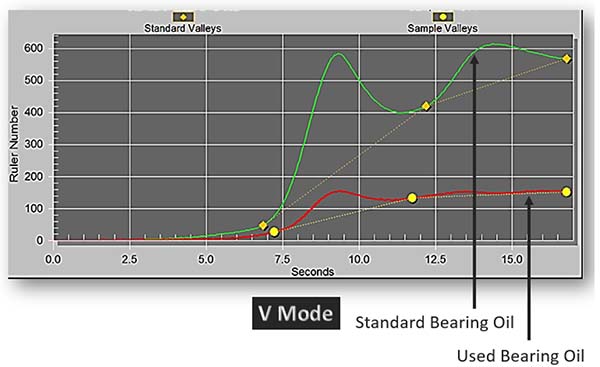
Figure 2: RULER graph of a standard bearing oil vs. used bearing oil.
Oxidation occurs worldwide on almost every item (food, the human body, and lubricants). It is not going away anytime soon. Hence, there will always be a need for antioxidants to help protect the base oils for finished lubricants.
However, the formulations of these antioxidants may evolve over time as scientists find new, more sustainable alternatives for creating antioxidants.
OEMs also have a role to play as they advance machines and their capabilities; lubricants will have to be engineered for these new applications with varying environmental conditions. As such, there may be a greater need for more advanced antioxidants to help protect the oil.
References
- Bruce, R. (2012). Handbook of Lubrication and Tribology Volume II Theory and Design. Boca Raton: CRC Press.
- (2022, July 20). Why Choose RULER? Retrieved from Fluitec: https://www.fluitec.com/why-choose-ruler/
- Livingstone, G. (2024, February 15). Varnish Deposits in Bearings, Causes, Consequences and Cures. Retrieved from Precision Lubrication Magazine: https://precisionlubrication.com/articles/varnish-deposits-in-bearings-causes-consequences-and-cures/
- Mang, T., & Dresel, W. (2007). Lubricants and Lubrication. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA.
- (2016, April 17). Oil Oxidation Stability Test. Retrieved from Mobil: https://www.mobil.com/en/lubricants/for-businesses/industrial/lubricant-expertise/resources/oil-oxidation-stability-test
- Mortier, R. M., Fox, M. F., & Orszulik, S. T. (2010). Chemistry and Technology of Lubricants. Dordrecht: Springer.
- Pirro, D. M., Webster, M., & Daschner, E. (2016). Lubrication Fundamentals, Third Edition, Revised and Expanded. Boca Raton: CRC Press.
- (2024, March 06). Oxidation, the oil killer. Retrieved from SKF: https://www.skf.com/us/services/recondoil/knowledge-hub/recondoil-articles/oxidation-the-oil-killer
- Spectro Scientific. (2024, March 06). Measuring Oil Chemistry: Nitration, Oxidation, and Sulfation. Retrieved from Spectro Scientific: https://www.spectrosci.com/en/knowledge-center/test-parameters/measuring-oil-chemistry-nitration-oxidation-and-sulfation
- Stachowiak, G. W., & Batchelor, A. W. (2014). Engineering Tribology. Butterworth-Heinemann.