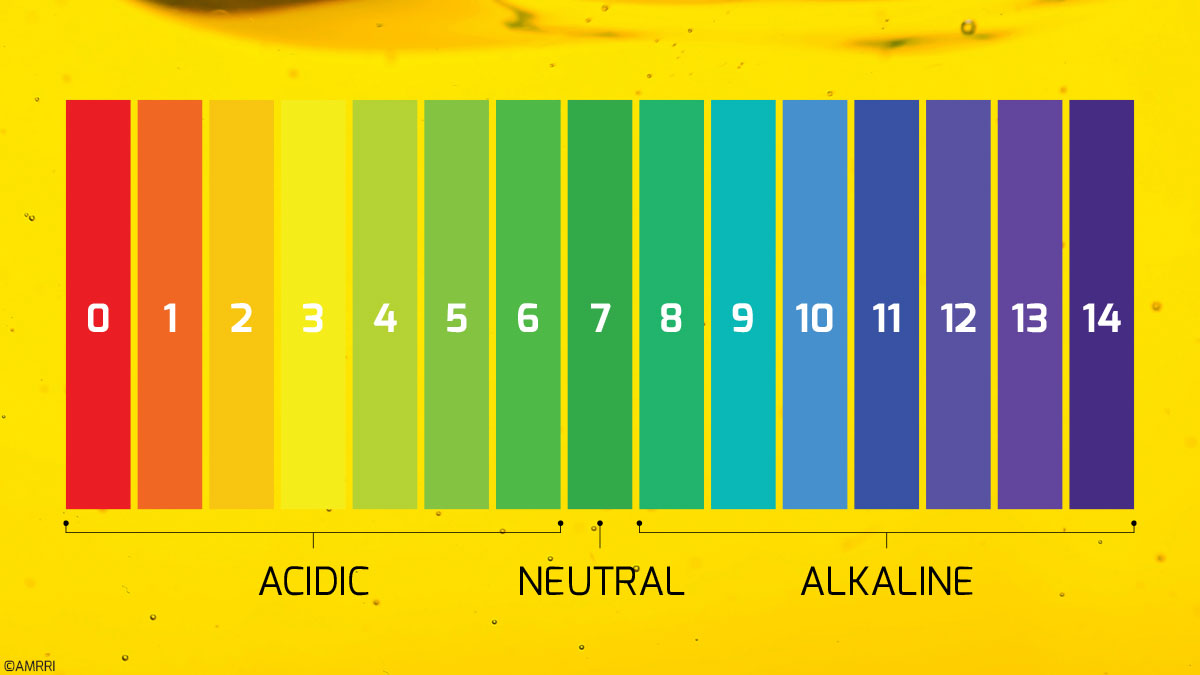
The Acid Number of a lubricant, sometimes called the “Total Acid Number” or TAN, is a commonly reported value for oil analysis and is generally considered an indication of oil aging and degradation.
But what does it mean, and how is it determined? Let’s break down what is reported, what techniques labs use, and if this parameter truly helps us pursue our goals for our lubricant monitoring programs.
The Mod. Squad
When I started my career at a U.S. Nuclear Power Plant in the late 1980s, I was hired as a recently degreed chemist to work in the facility’s maintenance department.
Admittedly an odd fit, but the wisdom of the move was to bring someone with some mechanical aptitude as an intern, and a chemistry background, into a maintenance department tasked with starting something called the “Predictive Maintenance Group.” On my first day, I was walked into a room full of laboratory instrumentation still in the shipping boxes and was told, “Here is an oil analysis laboratory. Go build it.”
That means there was no one to tell me, “This is how we have always done it.” Or point to an existing procedure or guide.
So I went to the ASTM Standards and created my own procedures. I was naïve enough to believe that that’s how everyone did oil analysis. So I was shocked when I visited a well-known and reputable laboratory to tour their facility. I noticed a jumbo box of paper clips beside the Acid Number titrator.
“What do you have these paperclips here for?” I asked.
I was told they were used as “disposable stirrer bars .” I was shocked, as I knew that the standard was very specific in the use of stirrers and the cleanliness required. I asked how they knew that the paperclips were sufficiently clean and consistent and how this met the requirements for the standard.
Next, I was told about a dirty little industry secret. The “mod.” designation. They explained that most of their ASTM standard methods were reported with the designation “mod.” for modification or modified, which means that the exact requirements of the standard were not followed but that a modified method was utilized to streamline the process to make it more economically competitive. And they explained that “Everybody does it. If we didn’t, we couldn’t perform the analysis cost-competitively.”
So what does that mean for the Acid Number test?
Is it essential for our laboratories to follow the standard precisely? Can the values possibly be different if they don’t? How do we know if a lab’s modifications to the standard method directly or potentially influence the measured parameters?
Unless auditing and periodic comparisons of measurements are made, such as the ASTM Proficiency Test Program, and evaluated for impact, the influence of such “shortcuts” might not be fully understood by the customer paying for the analysis.
How Is My Lab Testing for Acid Number?
If a customer submits samples to a lab for Acid Number analysis, you are probably like most and only get a single value returned. As a result, a quantity of potassium hydroxide per gram of oil, or mg KOH/g. Perhaps next to that is even an explainer of “D664” or maybe the dreaded “D664 mod.”
Does that help me understand what the lab did to arrive at that number?
When I began performing lubricating oil titrations, I utilized a Kyoto titrator to help automate the process per D664. Of course, instead of paper clips, I cleaned and dried Teflon stirrer bars for every titration and generated a titration curve for each sample.
Often, especially for the cleaner oils I tested, the titration curve was clear and produced two inflection points, one for weak and one for strong acids. Other times, there would be a glitch in the data near the inflection point, causing the automatic titrator to miss the endpoint, causing me to go back and manually calculate the endpoint.
The automatic titrator method allows the endpoint to be determined without an inflection point and taken as the value corresponding to a new value obtained every day in the lab using buffer solutions.
What about the volume of oil used to titrate?
The D664 method provides a table to determine the volume to be used and ranges from 20 grams of sample to just 0.1 grams. However, even for the most likely samples seen in an oil analysis lab, two very different titrations may be necessary, a low acid number value for new turbine and R&O oils of anticipated values <1.0 requiring 20 grams of sample, to the common additized gear oils that are between 1.0 and 5.0, requiring 5 grams of sample.
So, does my high-throughput lab check each sample and adjust titration volumes accordingly? Maybe they aren’t titrating my oil at all.
FTIR for Acid Number
With all of these complications, variations in method, and time-consuming steps to achieve an accurate titrated Acid Number, it is unsurprising that many labs have utilized Infrared Spectroscopy to determine the Acid Number value.
Initially, this approach to determining the Acid and Base numbers was pioneered at McGill University and involved a pre-treatment of the sample with a strong base or acid, and allow these to neutralize the acids and bases present in the used sample. An excess was used, and the leftover portion could be accurately quantified to determine how much acid or base had been in the sample.
However, today, few employ this method and instead utilize a chemometric approach to evaluate a used oil FTIR or I.R. spectrum and compare it to a reference spectrum. Assumptions are made about the types of acidic compounds that are typically formed and dominant in the used samples that have undergone oxidation. Calculations are made based on the height of peaks in these areas, correlating them to an Acid Number value.
If the assumptions about the source of acidity and oxidation hold, the Acid Number values reported in this way can be reasonably accurate. But they do not necessarily directly reflect the D664 titrated values that the used oil might produce. There are generally three ways to approach FTIR instead of chemical titration of used oil samples.
They are the Partial Least Squares method, a linear fit of the stoichiometry of prepared samples, or a combination of these approaches. The accuracy of these results depends on the vigor of the method used by the lab and the applicability of the references used to any particular sample that the lab subsequently encounters.
Some commercial labs have invested significant time in researching and developing methods to streamline the values produced by FTIR methods. Still, the customer that provides the sample is often unaware of how one lab’s techniques and approach may compare to another.
Is My Oil Becoming More Corrosive?
Even when we know that the test for Acid Number is being consistently performed and according to the applicable ASTM method, is there a complete understanding of what the Acid Number tells us?
I have seen, published in many places and presented by many experts over the years, explanations of the Acid Number or TAN, indicating that an increased value represents an increased “corrosive potential” of the lubricant.
My early attempts at introducing some new synthetic oil formulations to replace mineral oils in nuclear power plant components were initially rejected, citing that the new synthetic replacements had a higher Acid Number than the new oil data sheets for the existing mineral oils.
It was necessary to inform and teach the decision-makers that there was no connection between the Acid Number and the corrosive potential of the components in the machine.
This is outlined directly in the D664 standard, which states in the scope, “…the test method is not intended to measure an absolute acidic property that can be used to predict oil performance under service conditions. No general relationship between bearing corrosion and acid number is known.”
There are circumstances where the oil’s Acid Number is increasing, indicating increasing corrosive potential. That is when the oil is contaminated with strong and possibly mineral acids as part of the process near or in the machine or contaminants in the environment that the oil operates in.
Two examples might include diesel engines and specific compressors. In a diesel engine, the oil is initially formulated with an overbased detergent, with the understanding that fuel burning generates acidic byproducts that can build up in the oil over time. Initially, these are neutralized by the detergent, but eventually, the strong mineral acids may exceed the neutralization capacity and begin to build up in the oil.
This is why some use the testing strategy for both Base Number and Acid Number in combustion engines, looking for that point when the oil has lost its capability to keep the Acid Number low.
Another example would be natural gas compressors, mainly when the source gas is relatively “sour” or high in acidic content. As these gases would migrate into the compressor oil, Acid Numbers will rise sharply and reflect the increasing corrosive potential of these strong acids.
In some ways, I appreciated the former designation of TAN or Total Acid Number. It underscored the idea that this count included weak and strong acids. A significant increase in the Acid Number might reflect the addition of strong acids, a combination of strong and weak acids, or perhaps only weak acids (from hydrocarbon oxidation).
Without digging further, we don’t know, and the Acid Number is a fairly blunt tool for understanding what is happening regarding the chemistry of our oil.
Is Acid Number Even the Right Test?
After disconnecting the concept of measuring corrosion potential from the Acid Number, we return to the method’s original intent: to monitor the “…relative changes that occur in oil during use under oxidizing conditions…” (also from the D664 scope).
And this is indeed how many utilize Acid Number to monitor the oil’s degradation over time while in service, as evidence of its oxidation. However, this condition is primarily a lagging indicator.
In other words, the oil must undergo physical conversion from the original unoxidized hydrocarbon (typically) to an oxidized species that changes those molecules’ properties, including the ability to maintain a dynamic protective fluid film. As the Acid Number increases, the amount of molecules that have been compromised also increases.
However, the effect of potential damage to the machine in compromised lubricating films is not necessarily proportional to the increase in the Acid Number measuring this effect. Unfortunately, wear levels can start to increase before a substantial change is seen in the Acid Number, reflecting an accumulation of damaged molecules of lubricant.
If instead of targeting lubricant condition for the degraded ability to prevent damage, the desire is to prevent this degraded condition altogether, a leading indicator of degraded molecules would be required.
This is why in many monitoring strategies, other methods, such as the RULER technique (ASTM D7590), are utilized as a sign of the loss of the ability of the lubricant to prevent oxidation so that proactive measures can be made to replenish or replace the oil to reset the capability of the oil to resist oxidation.
In this regard, the Acid Number of the oil, concerning the degree of oxidation of the oil molecules, is similar to the “oxidation” parameter reported by Infrared Spectroscopy methods.
Infrared spectra are used to quantify the appearance of carbonyl groups, which are absent in many, mainly mineral, base oil formulations. The carbonyl groups can appear as carboylic acids, ketones, and aldehydes formed by the process of oil oxidation and are, therefore, a lagging indicator of the oxidation of viscous film-forming molecules in the lubricant.
So Where Do We Go From Here?
A review of any lubricant analysis program must start with the goals of the asset owner. Why am I performing Acid Number, how am I using the results, and what do I expect this investment in producing a trendable and comparable number will provide concerning my lubricant and my machine?
If we first ask these questions, we can then determine the initial decision point, which is whether or not the Acid Number adds any value to my lubricant analysis program.
If my goals are proactive, that I wish to detect the degradation of oils early and with enough time to act to avoid abnormal wear, oil degradation, and the formation of varnish, then Acid Number may not be the test to use.
In that case, leading indicator tests like RULER and MPC for varnish potential and large reservoir tests like RPVOT may be required and completely overtake the Acid Number from a lubricant management decision standpoint.
Work with your laboratory to better understand what tests and methods they use to provide you with an Acid Number and ensure they will help you meet your goals. An informed asset owner looks beyond a simple cost-per-sample approach and aligns their asset goals to the strategy of test slate selection and data result action.
References
Petrucci, R.H. and Wismer, R.K., General Chemistry with Qualitative Analysis, MacMillan Publishing Co., 1993.
McMurry, J., Organic Chemistry, Brooks/Cole, 1984
D664 – 18, “Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration”, ASTM, West Conshohocken, PA.